Parler
Parler Gab
Gab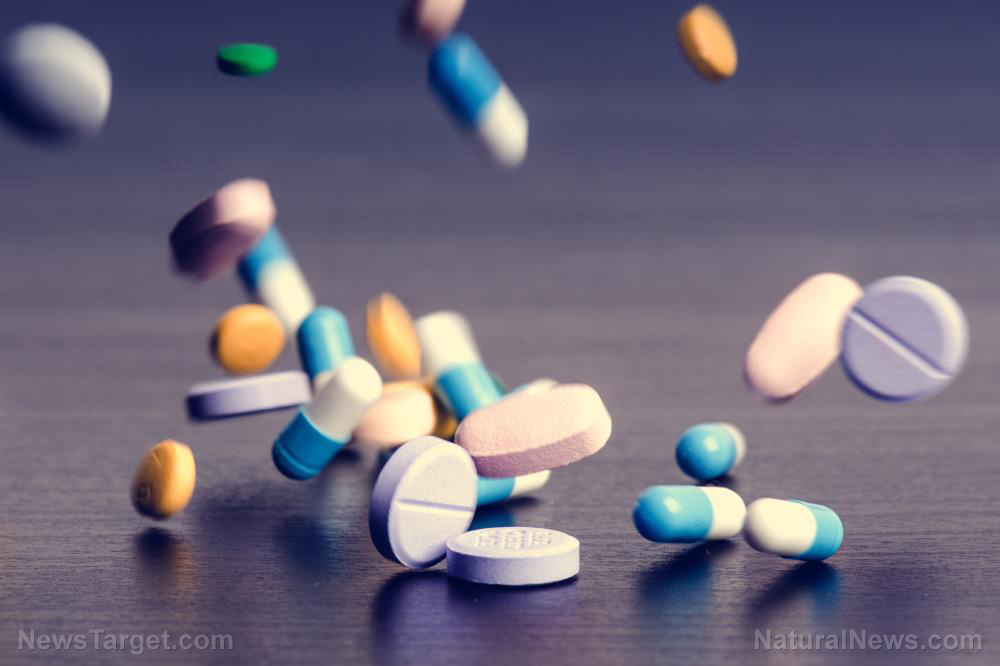
- Teva Pharmaceuticals recalled over 580,000 bottles of prazosin, a blood pressure and PTSD medication, due to contamination with cancer-causing nitrosamines.
- A 2025 study found generic drugs manufactured in India had 54.3 percent more serious adverse events than those made in advanced economies.
- The FDA inspects foreign drug manufacturing facilities less frequently and often with advance notice, unlike unannounced domestic inspections.
- Independent laboratories rather than regulatory agencies frequently discover dangerous contamination in medications.
- Patients and doctors have limited ability to identify where their generic drugs are manufactured or assess their quality.
The globalized drug supply chain
The transformation of pharmaceutical manufacturing over recent decades has been dramatic yet largely invisible to consumers. Today, approximately 80 percent of prescription drugs consumed in the United States originate from China and India, where production costs are significantly lower. This globalization occurred while the FDA maintained vastly different inspection standards for domestic and foreign facilities. For years, the agency operated on what critics describe as a "trust and not verify" approach toward overseas manufacturing, conducting far fewer inspections abroad despite the growing proportion of drugs produced there. The disparity became so pronounced that by 2010, 64 percent of foreign manufacturing plants had never received an FDA inspection, while U.S. facilities were examined approximately every three years. The Generic Drug User Fee Act of 2012 attempted to address this imbalance by requiring manufacturers to help fund inspections, yet the fundamental gap in oversight rigor remains. Foreign inspections are typically pre-announced with weeks of notice, unlike the surprise visits common domestically. Additionally, language barriers and reliance on manufacturer-provided translators potentially compromise these examinations. The consequences of this unfair, unequal system have been severe and sometimes fatal. In 2008, contaminated heparin from China resulted in over 100 deaths. In 2023, contaminated eye drops from India caused infections that led to 14 cases of vision loss, four surgical eye removals, and four deaths. Most recently, in 2024, extended-release potassium chloride capsules from Glenmark Pharmaceuticals in India released their entire dose at once rather than gradually, resulting in eight patient deaths before recalls were initiated.The discovery deficit
Perhaps most concerning is how rarely the FDA itself identifies these dangerous quality issues before they harm patients. The pattern has become distressingly familiar: independent laboratories, whistleblowers, or reports of patient harm typically trigger investigations rather than proactive regulatory oversight. Valisure, an independent testing laboratory, has repeatedly exposed contamination that eluded FDA detection. The lab first identified carcinogenic nitrosamines in widely prescribed blood pressure medications in 2019, then found the same contaminants in metformin, a common diabetes drug. Their testing later revealed excessive levels of the carcinogen benzene in popular consumer products including hand sanitizers, sunscreens, and acne treatments. In each case, the FDA responded to these discoveries rather than initiating them. The agency's reliance on voluntary adverse event reporting creates additional vulnerabilities. Unlike the mandatory reporting required for vaccines, clinicians face no such requirement for prescription drugs. Researchers estimate that fewer than 10 percent of adverse drug events are ever reported to the FDA. This underreporting creates a distorted safety picture that may leave dangerous medications on the market for years before patterns emerge. When the FDA does identify serious manufacturing problems, it often withholds crucial information from the public. Despite finding that Glenmark Pharmaceuticals' Indian facility had systematically falsified quality tests, the agency redacted the names of additional drugs manufactured at the same plant that had failed quality controls. This secrecy prevents pharmacies and consumers from avoiding potentially dangerous products, prioritizing manufacturer confidentiality over patient safety.The generic drug quality dilemma
The economic pressures of the generic drug market create perverse incentives that can compromise safety. When multiple manufacturers produce the same generic drug, competition focuses almost exclusively on price. Pharmacy benefit managers reimburse pharmacies at a fixed rate regardless of which generic version they dispense, creating powerful financial pressure to stock the cheapest options regardless of manufacturing quality. This system offers no reward for manufacturers who invest in superior quality control. In fact, it may punish them with higher costs that make their products less attractive to cost-conscious pharmacies. The situation becomes particularly problematic for older generic drugs like prazosin, which has been available for over 25 years. As more manufacturers enter the market, price competition intensifies, potentially encouraging cost-cutting measures that compromise quality. The FDA's "A-rated" system for generic drugs, intended to assure therapeutic equivalence between products, fails to distinguish between manufacturers with excellent quality records and those with repeated violations. Pharmacies and consumers have no straightforward way to identify where their generic drugs were manufactured or assess their quality beyond the binary "A" rating. This information asymmetry means that patients taking the same generic drug might have vastly different risk profiles depending on which manufacturer supplied their medication. The prescription pill that promises healing should not simultaneously threaten harm. Yet until the regulatory framework evolves to match the realities of globalized drug production, patients remain unwitting participants in a system where safety too often comes second to speed and savings. Sources include: MedicalXPress.com Journals.Sagepub.com CBSNews.com Forbes.comGovernments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By Patrick Lewis // Share
Ukrainian strikes, sanctions fuel global diesel crisis as prices soar
By Belle Carter // Share
Ultra-processed foods linked to surge in early-onset colorectal CANCER, study warns
By Patrick Lewis // Share
Social media use linked to lower reading and memory scores in children, new study finds
By Cassie B. // Share
By Lance D Johnson // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share